Discover Radiant Skin
About Us


Who We Are
PureKera
PureKera is located in Bozeman, Montana, specializing in the development and manufacture of sterile cell-free (acellular) amniotic fluid to support the body’s own skin renewal processes. The promise of sterile acellular amniotic fluid as an additive to moisturizers and gels to support healthy skin reparative processes is an exciting new approach to enhance aesthetic treatments. Medical spas and dermatologists are starting to use topical amniotic fluid to supplement treatments such as platelet-rich plasma facials, laser resurfacing and microneedling. Amniotic fluid supports the body’s healing for a faster recovery period and elicits no immune or rejection response in recipients due to the immune-privileged nature of amniotic tissues.
“The more you care about yourself makes you more beautiful.”
Who We Are
Company Background
The founders of PureKera have all worked in the regenerative biology and therapeutic fields for decades, as CEOs, Directors and Scientific Officers of human tissue banks and in research in transplantation, wound healing, stem cell and tissue engineering. Recent success in the utilization of human amniotic fluid and tissues in humans led the group to focus their efforts on developing superior amnion products for use in cosmetics.
Human acellular amniotic fluid is used by clinicians for a variety of medical conditions, including in several FDA-approved clinical trials to aid in healing of burn wounds,3,4 arthritis in joints, eye abrasions, chronic wounds, and other inflammatory mediated conditions.5 PureKera has case studies using bAF in more than 50 animals (equine, canine, seal, zebra, rhino, elephant) with no reported negative effects. Positive effects were most notable for supporting wound healing – cutaneous injuries and chronic non-healing wounds, eye lesions, osteoarthritis, tendonitis and lameness. Animal studies have shown the efficacy of bAF in treating skin wounds.6
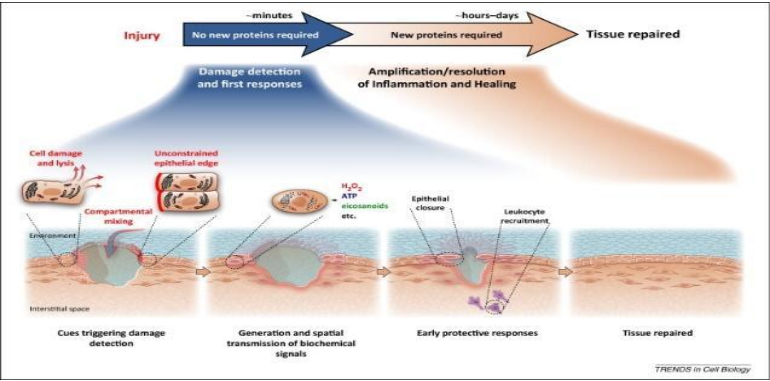
Amniotic fluid fills the amniotic membrane that surrounds the fetus in all mammalian species, protecting the fetus and providing myriad molecules that promote fetal growth and development. Acellular amniotic fluid contains hundreds of proteins affecting innate immune and inflammatory responses, including hyaluronic acid, cytokines, chemokines, growth factors, antibacterial peptides and anti-inflammatory molecules and exosomes.1, 2
PureKera has developed a cosmetic product that utilizes acellular bAF. PureKera applies state-of-the-art manufacturing using proprietary processes. PureKera’s bAF is sterile filtered, cryopreserved and kept frozen until formulated into cosmetic products, maintaining beneficial protein levels using industry standards and procedures. To date no bAF harvested and processed at the PureKera facility has tested positive for any viruses (pre-processing) or any bacteria/fungi (post processing). PureKera’s bAF has been tested in several species, including humans, and found to be effective in supporting the body’s own skin reparative and protective processes. Microarray analysis of PureKera’s bAF revealed rich amounts of cytokines and chemokines associated with wound healing immunomodulatory and anti-inflammatory activity.
References
- Jan Pierce, Pam Jacobson, Eric Benedetti, Emily Peterson, Jessica Phibbs, Amber Preslar, JoAnna Reems. Collection and characterization of amniotic fluid from scheduled C-section deliveries. July 2016. Cell Tissue Bank. DOI 10.1007/s10561-016-9572.
- Yong Mao, Jan Pierce, Anya Singh-Varma, Michael Boyer, Joachim Kohn and Jo-Anna Reems. Processed human amniotic fluid retains its antibacterial activity. Mao et al. J Transl Med (2019) 17:68. https://doi.org/10.1186/s12967-019-1812-8
- Anna M Darelli-Anderson, BA, Samuel South, MD, Giavonni M Lewis, MD, FACS, 557 Amniotic Fluid Injections in Chronic Non-Healing Wounds in Pediatric Patients: A Case Series, Journal of Burn Care & Research, Volume 42, Issue Supplement_1, April 2021, Pages S127–S128, https://doi.org/10.1093/jbcr/irab032.207
- Topical Application of Purified Amniotic Fluid Accelerated Healing of Full-Thickness Burns, Negating the Need for Skin Grafts: A Case Report. Mercedes F. Kweh et al. Journal of Wound Management and Research 2023; 19(1): 53-58
- Bowen, C.M.; Ditmars, F.S.; Gupta, A.; Reems, J.-A.; Fagg, W.S. Cell-Free Amniotic Fluid and Regenerative Medicine: Current Applications and Future Opportunities. Biomedicines 2022, 10, 2960. https://doi.org/10.3390/biomedicines10112960
- Low-Level Laser and Bovine Amniotic Fluid-derived Cream Accelerating Skin Neck Wound Healing and Reducing Inflammation and Wound Scar in a Rat Animal Model. Abbasiazar, Davoud et al. Journal of Cutaneous and Aesthetic Surgery 15(3): p 267-274, Jul–Sep 2022. | DOI: 10.4103/JCAS.JCAS_79_22
Why Choose Us
Why We Are the Best


- State-of-the-Art Manufacturing: Our proprietary processes ensure unparalleled purity and efficacy.
- Scientific Validation: Backed by extensive research and real-world case studies.
- Commitment to Safety: Rigorous testing guarantees the highest standards of product quality.
- Sustainability: Ethical sourcing and innovative practices define our approach.
Meet Our Experts
Meet the founders and the masterminds behind the science.

Jan Pierce, MBA
Founder, President/Chief Financial Officer
Mr. Jan Pierce has been working in healthcare, human cell and tissue industry for over 40 years. He has significant experience and knowledge in cGMP manufacturing, FDA regulations for human cells, tissues and medical devices. He co-developed four cell and tissue products, one cell culture supplement and a tissue packaging system for storage of autologous tissues (BioSecure®) owning patients and trademarks. He has held various Board, Council and Committee positions at the American Association of Tissue Banks (AATB), and Salt Lake County Sheriff’s Search and Rescue. He has written standards for AATB including skin, amniotic membrane and amniotic fluid. He has developed processes and SOPs for cell and tissue collection, processing, testing, storage, packaging, labeling, quality control, quality assurance and distribution. He is the author of several publications and book chapters. He is the Founder, President and CEO of Bio Cell and Tissue Technologies, Inc. (BIOCETT). He holds a Senior Director leadership position at the University of Utah School of Medicine’s Cell Therapy and Regenerative Medicine Department.

Jane Shelby, Ph.D.
Founder, Chief Scientific Officer
Dr. Shelby brings extensive experience in the study of transplantation and trauma immunobiology, wound healing research and tissue regeneration. She obtained her doctorate at the University of Utah, and spent many years there involved in medical research experience as a tenured Associate Professor of Surgery. She held the positions of Scientific Officer of the Intermountain Tissue Center in Utah, and Senior Vice President of Research at Bacterin International in Belgrade MT. Dr. Shelby leverages this experience to expand regenerative products. She recently retired as the Director of the University of Alaska Anchorage WWAMI School of Medical Education, and Regional Dean at the University of Washington School of Medicine. She is an Affiliate Professor of Medical Education and Biomedical Informatics at the University of Washington School of Medicine, and lives in the mountains outside Bozeman, Montana.

Darrel Holmes
Founder, Chief Operating Officer, CTBS
Mr. Holmes has over 40 years of experience in the medical device, biologics, tissue banking, and diagnostic industries. Mr. Holmes is a founder of Horizon Biologics and PureKera Cosmetics and serves as their COO. He also serves as KDNA Life Sciences’ Tissue Bank Director and on their Medical Advisory Committee. He previously served as Operations Executive for American Qualex, HYCOR Biomedical and Stratagene Cloning Systems, Big Spring Water Company, and Xtant Medical (formerly Bacterin International). Mr. Holmes has had responsibilities for all aspects of medical device and biologic product design and development, process scale-up, and production. He was also responsible for the establishment and maintenance of an FDA CFR Title 21 Part 820- and Part 1271-compliant quality system and has worked with numerous regulatory agencies at the federal, state, and local level. Mr. Holmes has implemented and managed companies’ ISO 13485 and ISO 17025 compliance and environmental health and safety programs. He has been the primary regulatory interface for several companies and managed operations including production, regulatory submissions, technology transfer, facility and engineering management, to produce medical devices and biologic products.